
The right support for every critical decision
Every day, you and your teams face increasing demands: rising patient volumes, staffing shortages, tighter budgets, and the pressure to deliver advanced care outside the hospital. Critical decisions must be made in seconds, and delays can cost lives.
Our solutions
Certified quality you can trust
Our products are registered under UK’s Medicines and Healthcare products Regulatory Agency (MHRA) for market placement, and aligned with Cyber Essentials, the UK government–backed cybersecurity scheme that strengthens organisational resilience. We are also registered in the WAND database in New Zeeland. In Australia we are part of the Australian Register of Therapeutic Goods (ARTG) under the Therapeutic Goods Administration (TGA).
- ISO-Certified: We are certified by Intertek to ISO 13485, ISO 27001, and ISO 20000-1, demonstrating our commitment to continuous improvement and the delivery of products and services of the highest quality. These certifications give our customers, partners, and suppliers confidence that our solutions are safe, reliable, and consistently performant.
- Medical Device Regulation (MDR) Compliance: MobiMed is classified as a Class IIb medical device under EU MDR, ensuring it meets the strict safety and quality standards required for manufacture, import, and sale within the EU. Every CE-marked product reflects our dedication to protecting patients and supporting healthcare teams with trusted, compliant tools.
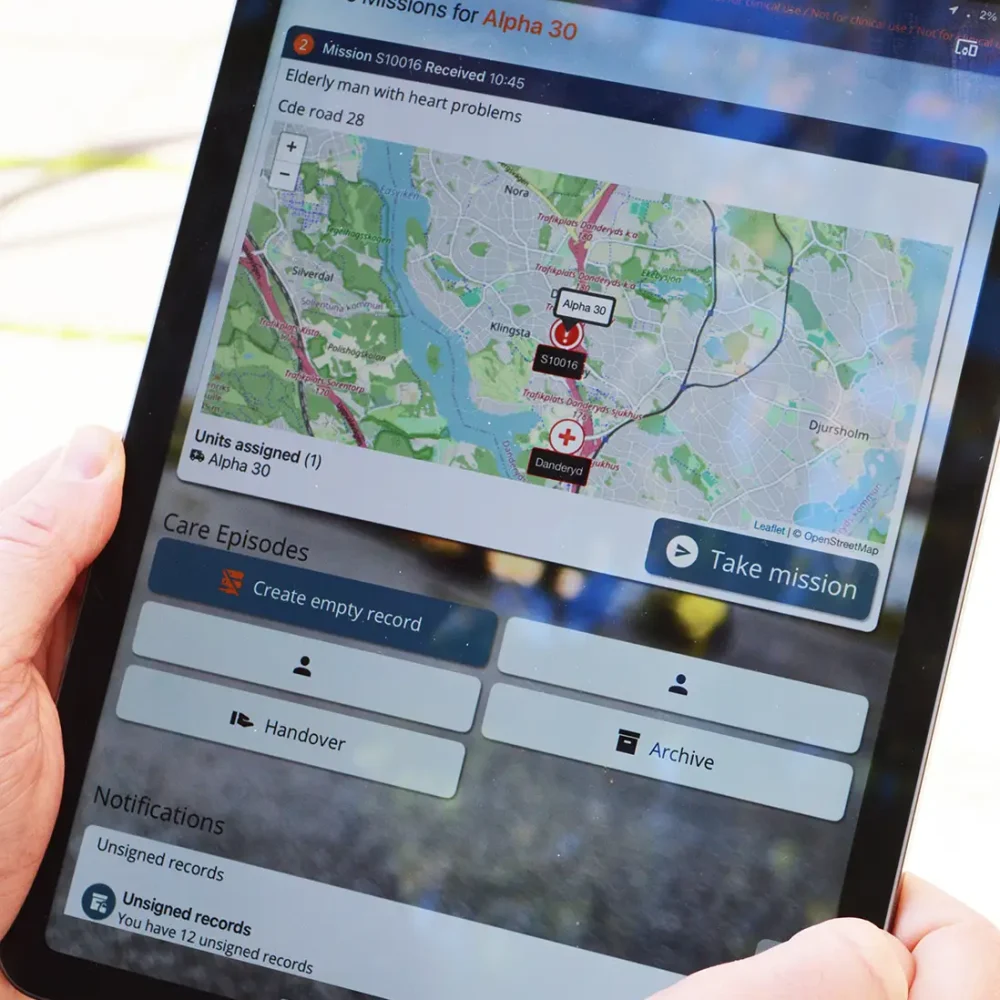

Why choose Ortivus?
Because we understand your challenges:
- You need more capacity outside hospitals. We provide cost-effective digital solutions to treat patients closer to home.
- You need to reduce unnecessary admissions. Our decision support helps clinicians diagnose and treat on scene.
- You need safe, reliable systems. We deliver robust, field-tested solutions trusted by thousands worldwide.
With us, you get a partner focused on helping you deliver safer care, use resources more effectively, and support your teams in every mission.
